Background. IPSSWM and revised-IPSSWM are the two currently prognostic scores used for survival prediction in symptomatic Waldenström Macroglobulinemia (WM) patients in need of treatment. In addition to age and serum beta-2 microglobulin levels, which are present in both scores, the IPSSWM includes anemia, thrombocytopenia and the concentration of the serum monoclonal component (MC), while revised-IPSSWM includes LDH and serum albumin levels. Both IPSSWM and revised-IPSSWM have been developed in retrospective series and apply only to symptomatic WM patients.
Aim. To analyze WM patients prospectively enrolled in the NF10 observational study since 2010 with the aim to define a prognostic score applicable to all WM patients at diagnosis, either symptomatic or asymptomatic.
Methods. The progression-free survival (PFS) and overall survival (OS) rate was estimated and plotted using the Kaplan-Meier method, with 95% confidence interval (95% CI), and the effect of covariables on PFS was estimated by means of the Cox proportional hazard (PH) regression. The association with PFS was expressed as hazard ratio (HR) with 95% CI, either in univariable or multivariable regression. All statistical tests were two-sided. The proportionality of risk of the multivariable Cox PH model was checked graphically by means of the scaled Schoenfeld residuals, and the model performance was analyzed using the likelihood displacement method. Starting from a model with 10 variables selected on the basis of clinical and statistical considerations, the final model was selected on considering the Akaike (AIC) and likelihood ratio test. The cut-offs were selected after modelling the covariate with restricted cubic spline in Cox regression. The final model was internally validated using bootstrap techniques to evaluate the discriminant power (C-index), slope shrinkage to check for overfitting, and calibration (comparison between predicted and actual survival).
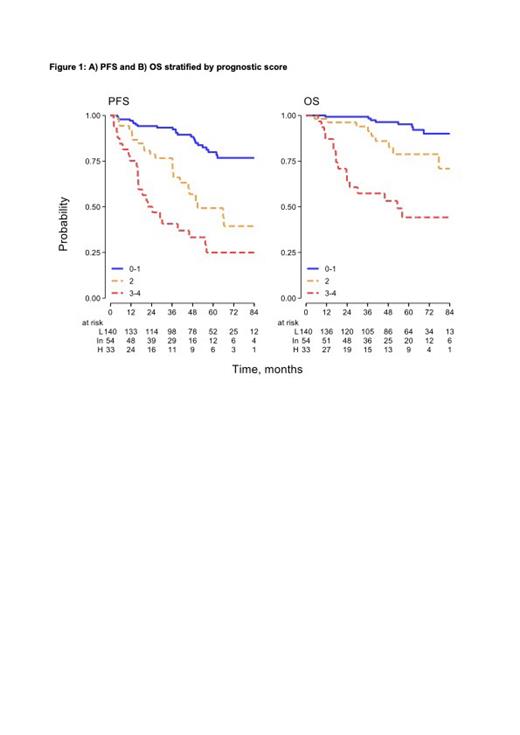
Results. We identified 303 eligible patients from the NF10 study, of whom 185 (60%) needed treatment immediately after diagnosis (42%) or later (18%). These were main patients characteristics: 59% were male, 46% were aged >70 years, 8% had constitutional symptoms, 55% had Hb <12 g/dL, 54% had an absolute lymphocyte count ≥2x10 9/L, 9% had platelets <100x10 9/L, 37% had a serum MC >2 g/dL, 21% had albumin <3.5 g/dL, 23% had LDH > upper limit of normal (ULN), 32% had beta-2 microglobulin >1.5xULN. With a median follow-up of 51 months, 5-year PFS for the entire cohort was 67% (95%CI 60-73%). In univariate analysis, age >70 years (P=0.043), constitutional symptoms (P=0.009), nodal sites (P=0.002), Hb <12 g/dL (P<0.001), absolute lymphocyte count ≥2x10 9/L (P=0.033), serum MC >2 g/dL (P=0.003), high beta-2 microglobulin (P<0.001) and LDH (P=0.001), albumin <3.5 g/dL (P<0.001), C-reactive protein >4 mg/dL (P=0.008) were associated with shorter PFS. In multivariate analysis, age, anemia, hypoalbuminemia and high LDH retained prognostic value and thus entered the final model. Patients were classified into 3 risk groups: low (0-1 factors, 62% of patients), intermediate (2 factors, 24%) or high (3-4 factors, 15%), with a 5-year PFS of 80% (95%CI 70-87%), 49% (95%CI 32-64%) and 25% (95%CI 11-42%), respectively, with C-Harrell=0.744 and robust internal validation and calibration. OS was respectively 96% (95%CI 90-98%), 77% (95%CI 62-87%) and 43% (95%CI 25-60%) for the low, intermediate and high risk group (Figure 1).
Conclusions. We developed a prognostic model based on 4 parameters easily attainable in clinical practice, able to predict PFS and OS at diagnosis. This is the first prognostic score for WM developed in a prospective study and applicable to patients presenting either with symptomatic or asymptomatic disease. This prognostic model identifies a subset of high-risk patients with a dismal outcome and a median OS <5 years. External validation in independent cohorts is needed.
Disclosures
Varettoni:ASTRAZENECA: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BEIGENE: Honoraria, Membership on an entity's Board of Directors or advisory committees; JANSSEN: Honoraria, Membership on an entity's Board of Directors or advisory committees; ABBVIE: Honoraria, Membership on an entity's Board of Directors or advisory committees. Ferrero:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy; Sandoz: Consultancy; Beigene: Research Funding; Morphosys: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees; Clinigen: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gentili: Speakers Bureau; Italfarmaco: Membership on an entity's Board of Directors or advisory committees. Merli:Roche: Other: advisory board; Gilead: Other: advisory board; Novartis: Other: advisory board; Takeda: Other: advisory board; Incyte: Other: advisory board; Janssen: Other: advisory board; MSD: Other: advisory board. Pulsoni:Roche: Consultancy, Honoraria, Speakers Bureau; MSD: Honoraria, Speakers Bureau; SANDOZ: Honoraria, Speakers Bureau; TAKED: Consultancy, Honoraria, Speakers Bureau; GILEAD: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Honoraria, Speakers Bureau; JANSSEN: Honoraria. Visco:AbbVie, Lilly, BMS, Astra Zeneca, Servier, Incyte, Roche, Pfizer, Novartis, Gentili, Janssen, Kite-Gilead, Beigene: Honoraria, Speakers Bureau; AbbVie, BMS, Incyte, Roche, Pfizer, Janssen, Lilly: Membership on an entity's Board of Directors or advisory committees. Annibali:Amgen: Speakers Bureau; Janssen: Speakers Bureau; Takeda: Speakers Bureau. Ferreri:Adienne: Speakers Bureau; ADC Therapeutics, Amgen, BeiGene, BMS, Genmab, Gilead, Hutchison Medipharma, Novartis, Pharmacyclics, PentixaPharm, Pfizer, Roche: Research Funding; Ospedale San Raffaele srl.: Patents & Royalties; Gilead, Incyte, Novartis, PentixaPharm, Roche: Consultancy. Luminari:Regeneron Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal